Your cart is currently empty.
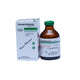
Sunny D Insta 5 mg / 1 ml Injection – Vitamin D3 Ampoule
: Available:
QUESTIONS & ANSWERS
Have a Question?
Be the first to ask a question about this.
Category:

Description
Sunny D Insta injection is a high-dose Vitamin D3 (cholecalciferol) formulation used to treat or prevent vitamin D deficiency, especially when oral administration is not feasible or in cases of malabsorption. Each 1 ml ampoule contains 5 mg (200,000 IU) of Vitamin D3.
Ingredients
-
Active ingredient: Cholecalciferol (Vitamin D3), 5 mg per 1 ml ampoule (~200,000 IU)
-
Inactive / excipients: (manufacturer’s formulation including solvent, stabilizers, etc.) — check official leaflet.
Drug Class
Vitamin D analog / Nutritional supplement / Fat-soluble vitamin
Dosage Form
Injection (sterile solution) — 1 ml ampoule containing 5 mg cholecalciferol (Vitamin D3)
Uses (Indications)
Sunny D Insta is used for:
-
Correction of Vitamin D deficiency, especially in patients who cannot take oral supplements
-
Cases of malabsorption, chronic liver disease, or when oral uptake is compromised
-
Support for bone health, calcium metabolism, and maintenance of normal vitamin D levels
Dosage
-
The dose is high (5 mg / 200,000 IU) in a single ampoule; the exact regimen or frequency is determined by the physician based on the patient’s deficiency severity, baseline vitamin D levels, and risk factors.
-
Such high-dose injections are typically not repeated frequently; follow monitoring guidelines (e.g. checking serum 25(OH)D, calcium) before further dosing.
In Case of Overdose
-
Excessive vitamin D can lead to hypercalcemia — symptoms may include nausea, vomiting, weakness, frequent urination, kidney stones, confusion.
-
Management: Stop supplementation, restrict dietary calcium, hydrate, monitor and treat hypercalcemia under medical supervision.
Missed Dose
-
In a clinical setting, missing an injection should be addressed by the treating physician.
-
Do not self-administer or double the dose to compensate.
How To Use / Administration
-
Administer via intramuscular (IM) injection (often recommended for depot effect) or as per manufacturer instructions.
-
Use aseptic technique.
-
Monitor patients during and after injection for adverse events, especially in cases of underlying conditions (kidney, calcium metabolism).
When Not to Use (Contraindications)
-
Hypercalcemia or hypervitaminosis D
-
Severe renal impairment or nephrolithiasis
-
Known hypersensitivity to vitamin D or any component of formulation
-
Disorders of calcium metabolism (unless under careful monitoring)
-
Use with caution in pregnancy or lactation — only if clearly needed
Side Effects
Common / Less serious:
-
Mild hypercalcemia symptoms (thirst, urination)
-
Gastrointestinal discomfort
-
Headache
Less common / Serious:
-
Hypercalcemia and associated complications (renal stones, bone pain)
-
Soft tissue calcification
-
Allergic reactions
Precautions & Warnings
-
Monitor serum calcium, phosphate, magnesium after injection
-
Check renal function
-
Avoid excessive concurrent intake of calcium or vitamin D
-
Be cautious in patients with cardiovascular disease, kidney disease, or predisposition to hypercalcemia
-
Adjust or avoid in patients using thiazide diuretics (which can increase calcium reabsorption)
Drug Interactions
-
Thiazide diuretics — may potentiate hypercalcemia
-
Digoxin — altered calcium levels may affect cardiac rhythm
-
Magnesium supplements, calcium supplements — risk of additive hypercalcemia
-
Some anticonvulsants (e.g. phenobarbital, phenytoin) — may increase vitamin D metabolism
Storage / Disposal
-
Store at controlled room temperature, away from light, heat, and moisture (often ≤ 30 °C)
-
Do not freeze
-
Use before expiration
-
Dispose unused ampoules according to medical waste regulations
Control Drug Status
-
Not a controlled substance; it is a prescription / professional-use medication
Quick Tips
-
High-dose injections are tools for repletion, not for frequent long-term use
-
Always monitor calcium, vitamin D levels, and kidney function post-administration
-
Avoid combining with high calcium supplements immediately
-
Be alert for symptoms of hypercalcemia
-
Use under medical supervision, particularly in patients with comorbid conditions
Your order of 100$ or more gets free standard delivery.
- Standard delivered 4-5 Business Days
- Express delivered 2-4 Business Days
Orders are processed and delivered Monday-Friday (excluding public holidays)
eMarket members enjoy free returns.
Related Products

Categories
Custom HTML Text
-
Free Delivery
From Rs 5000
-
Support 24/7
Online 24 hours
-
Free return
365 a day
- Choosing a selection results in a full page refresh.
Added to cart successfully. What's next?

Product type: 1
1 x $00.00





















 Chat with Us
Chat with Us