Your cart is currently empty.
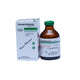
Transamin 250 mg / 5 ml Injection – Box of 10 Ampoules (Tranexamic Acid Antifibrinolytic)
: Available:
QUESTIONS & ANSWERS
Have a Question?
Be the first to ask a question about this.
Category:

Description
Transamin injection is a parenteral form of tranexamic acid, an antifibrinolytic agent. It works by inhibiting the breakdown of blood clots (fibrinolysis), thereby helping to control or prevent excessive bleeding. This injectable form is used in acute or perioperative situations under medical supervision.
Ingredients
-
Active ingredient: Tranexamic acid (250 mg per 5 ml)
-
Inactive / excipients: Water for injection, buffering agents, stabilizers (depending on manufacturer)
Drug Class
Antifibrinolytic / Hemostatic agent (coagulation modifier)
Dosage Form
Sterile injectable solution — 5 ml ampoule delivering 250 mg of tranexamic acid
Uses (Indications)
Transamin injection is indicated in conditions where there is excessive fibrinolysis and risk of bleeding, including:
-
Controlling bleeding in surgical or dental settings (especially in patients with bleeding risk)
-
Preventing or treating hemorrhage in hyperfibrinolytic states
-
Heavy bleeding from gynecological sources or menorrhagia (as adjunct)
Dosage
-
The specific dose depends on the clinical setting, severity of bleeding, and patient’s condition (e.g. weight, renal function).
-
In many clinical protocols, tranexamic acid injections are dosed based on mg per kg of body weight administered IV.
-
For example, in dental extraction for patients with bleeding disorders, 10 mg/kg may be used IV before and after procedure.
-
Because 250 mg / 5 ml is a relatively lower dose form, dosing adjustments or combining ampoules might be required depending on clinical need and physician orders.
In Case of Overdose
-
Symptoms: nausea, vomiting, diarrhea, dizziness, hypotension, risk of thromboembolic events, seizures in severe cases.
-
Management: supportive treatment, monitor vital signs, symptomatic care, possibly seizure management.
Missed Dose
-
In hospital/clinical settings with injections, the concept of “missed dose” is less applicable to patient self-administration.
-
If a scheduled injection is missed, follow the plan by the treating physician—do not self-administer extra dose.
How to Use / Administration
-
Administer via intravenous (IV) route (preferred) under aseptic conditions.
-
Infuse or inject carefully: when undiluted, limit rate (e.g. ≤ 1 mL/min) to avoid hypotension.
-
Some protocols allow dilution in compatible IV fluids before infusion.
-
Monitor the patient’s vital signs, bleeding control, renal function, and signs of adverse effects during and after administration.
When Not to Use (Contraindications)
-
Hypersensitivity to tranexamic acid or any component of formulation
-
Active intravascular clotting, e.g. DVT, pulmonary embolism, or thromboembolic disease
-
Hematuria (risk of blocking urinary tract)
-
Severe renal impairment (risk of accumulation) unless dose adjusted
-
Intrathecal or epidural use (incorrect route) is contraindicated and has caused serious toxicity (neurologic events)
Side Effects
Common / Less Serious:
-
Nausea, vomiting, diarrhea
-
Dizziness, lightheadedness
-
Hypotension (especially if injected too fast)
-
Skin reactions, rash, itching Drugs.com
Serious / Rare:
-
Seizures (especially with high doses or inadvertent neuraxial administration)
-
Thromboembolic events (clot formation) in predisposed patients
-
Visual disturbances, retinal changes (rare and dose dependent)
-
Allergic reactions, anaphylaxis (rare)
Precautions & Warnings
-
Monitor renal function; adjust dose in renal impairment
-
Use lowest effective dose for shortest duration
-
Caution in patients with history of thromboembolic disease
-
Avoid combining with other prothrombotic agents unless under supervision
-
Be cautious in patients with seizures or risk thereof
-
Discontinue use if signs of visual disturbances or changes in vision occur
Drug Interactions
-
Use with other procoagulant agents or hormonal therapies may increase clot risk
-
Use caution when co-administering with anticoagulants or fibrinolytic agents
-
No major interactions are well established beyond clotting-related risk
Storage / Disposal
-
Store at room temperature, protected from light and moisture
-
Do not freeze
-
Use before expiry date
-
Dispose of unused or expired ampoules according to medical / pharmaceutical waste guidelines
Control Drug Status
-
Not a controlled narcotic substance, but a prescription-only medication requiring medical oversight
Quick Tips
-
Administer slowly IV to reduce risk of hypotension
-
Monitor for signs of clotting, especially in high-risk patients
-
Adjust dose in renal impairment
-
Avoid use in patients with a history of thrombosis unless necessary and monitored
-
In case of neurologic symptoms, discontinue and seek medical help
Your order of 100$ or more gets free standard delivery.
- Standard delivered 4-5 Business Days
- Express delivered 2-4 Business Days
Orders are processed and delivered Monday-Friday (excluding public holidays)
eMarket members enjoy free returns.
Related Products
Categories
Custom HTML Text
-
Free Delivery
From Rs 5000
-
Support 24/7
Online 24 hours
-
Free return
365 a day
- Choosing a selection results in a full page refresh.
Added to cart successfully. What's next?

Product type: 1
1 x $00.00



















 Chat with Us
Chat with Us