Your cart is currently empty.
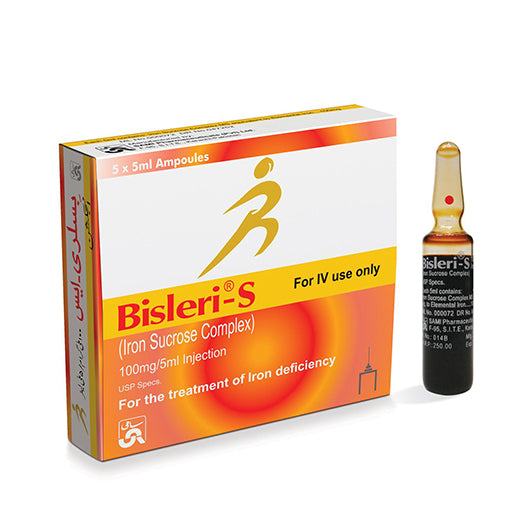
Bisleri-S 100 mg / 5 mL IV Iron Sucrose Injection – Box of 5 Ampoules
: Available:
QUESTIONS & ANSWERS
Have a Question?
Be the first to ask a question about this.
Category:

Description
Bisleri-S is an intravenous (IV) iron preparation containing iron sucrose complex. It is used to treat iron deficiency anemia (especially when oral iron is not feasible or poorly tolerated).
It comes as 5 mL ampoules, each containing 100 mg elemental iron in 5 mL solution (i.e. 20 mg/mL).
A typical pack may contain 5 ampoules of 5 mL each.
Manufacturer / Brand: Sami Pharmaceuticals (Pvt.) Ltd. (Pakistan)
Ingredients
-
Active ingredient: Iron Sucrose Complex equivalent to 100 mg elemental iron in 5 mL
-
Excipients: (Not always publicly disclosed in product listing) Typical excipients in iron sucrose injections may include water for injection, buffer agents, stabilizers, but you should verify from the product’s official brand / leaflet.
Drug Class
-
Hematinic / Iron preparation
-
Anti-anemic agent
-
Intravenous iron therapy
Dosage Form
-
Solution for intravenous use
-
5 mL glass ampoule
-
Sterile, for injection only
Uses / Indications
Bisleri-S (iron sucrose) is indicated for:
-
Treatment of iron deficiency anemia (IDA) when oral iron is ineffective, not tolerated, or during periods of increased need
-
Correction of iron deficiency in patients with chronic kidney disease (especially those on hemodialysis)
-
Perioperative or postoperative iron supplementation when significant blood loss occurs and rapid iron repletion is needed
-
Situations where oral iron absorption is impaired (e.g., malabsorption, inflammatory bowel disease)
-
In surgical procedures to maintain or restore iron levels and reduce need for transfusion
Note: The exact labeled indications may vary by country / regulatory agency.
Dosage & Administration
Typical dosage
-
The common dose is 100 mg elemental iron (i.e. 5 mL) per administration
-
It may be given undiluted slowly (over 2 to 5 minutes) or diluted in compatible infusion fluid (e.g. up to ~100 mL of 0.9% NaCl) depending on institutional protocol.
-
The total course may require multiple doses spaced out (e.g. every few days), until total iron deficit is replaced. The cumulative dose depends on the patient’s hemoglobin level, body weight, and iron deficit.
Always follow the prescribing / hospital protocol and local regulatory labeling.
In Case of Overdose
-
Overdose of intravenous iron (especially large bolus) can result in iron toxicity, which may manifest as hypotension, tachycardia, nausea, vomiting, diarrhea, metabolic acidosis, shock, and multi-organ failure in extreme cases.
-
Management is supportive. There is no specific antidote; chelation therapy (e.g. with deferoxamine) may be considered in severe cases under medical supervision.
-
Monitor vital signs, renal and hepatic function, electrolytes.
Missed Dose
Because iron sucrose is not usually given on a strict daily schedule (but rather on scheduled appointments / infusions), the concept of a “missed dose” is less typical than for oral medications. If a scheduled infusion is missed, it should be given as soon as possible, considering patient safety (e.g. allow adequate observation time) and according to physician instructions.
How to Use / Administration Instructions
-
Verify the patient, dosage, and protocol.
-
Inspect the ampoule solution: it should be clear to slightly brownish; no particulate matter or discoloration.
-
Use aseptic technique to open the ampoule.
-
Either administer slowly undiluted (2–5 min) or dilute in compatible IV fluid (e.g. 0.9% sodium chloride) up to an acceptable volume (per institutional guidelines).
-
Monitor patient during and after infusion for signs of hypersensitivity or reactions (vital signs, signs of anaphylaxis).
-
Use appropriate infusion set / filters (if required).
-
Dispose of ampoules / sharps according to biomedical waste guidelines.
When Not to Use / Contraindications
-
Known hypersensitivity or allergic reaction to iron sucrose or any component of the formulation
-
Iron overload conditions (e.g. hemochromatosis, hemosiderosis)
-
Active severe infections (because iron may support microbial growth)
-
Uncorrected anemia of other causes not iron deficient
-
First trimester of pregnancy (in some protocols) or when risk vs benefit not favorable (check local label)
-
Caution in patients with a history of reactions to parenteral iron
-
Use with caution in patients with liver disease, peptic ulcers, or gastrointestinal bleeding.
Side Effects / Adverse Reactions
Common / less severe:
-
Nausea
-
Vomiting
-
Headache
-
Hypotension (especially if administered too rapidly)
-
Flushing
-
Muscle cramps
-
Local irritation at injection site
-
Dizziness
Less common / serious:
-
Hypersensitivity reactions / anaphylaxis
-
Hypotension severe
-
Iron overload (if excess dosing)
-
Arthralgia, myalgia
-
Injection-site reactions
-
Dyspnea, wheezing in allergic reaction
-
Skin reactions
If any signs of serious reactions occur (e.g. rash, swelling, difficulty breathing, hypotension), stop administration immediately and manage medically.
Precautions & Warnings
-
Always administer under supervision of trained personnel with resuscitation facilities available for hypersensitivity / anaphylactic reactions.
-
Test dose: Some clinicians administer a test / diluted small dose first to check for hypersensitivity (depending on label / local protocol).
-
Monitor for signs of iron overload, especially in repeated dosing.
-
In patients with infection, iron administration should be carefully considered because pathogens may use iron.
-
Use caution in patients with hepatic dysfunction, as iron is stored in liver.
-
Monitor hemoglobin, ferritin, transferrin saturation, and iron indices before and after therapy.
-
Monitor vital signs during infusion.
-
Use caution in patients with asthma or other allergic predisposition.
-
Avoid coadministration with other parenteral iron preparations unless protocol supports.
Drug Interactions
-
Some antibiotics and anti-cancer drugs may have altered effects in presence of free iron — check specific interaction references.
-
Concurrent use of chelators (e.g. deferoxamine) may reduce iron effect.
-
Iron may interfere with absorption of oral tetracyclines, fluoroquinolones, certain oral drugs if given concurrently (though this is more relevant to oral iron).
-
Concurrent administration of other parenteral iron products should be avoided unless under specialist direction.
Always check a drug interaction database or local formulary for up-to-date interactions.
Storage / Disposal
-
Storage: Store ampoules at 2 °C to 8 °C (refrigerated). Protect from light and freezing.
-
Do not use if ampoule is broken, cracked, discolored, or contains particulate matter.
-
Follow “first-in, first-out” principle with stock.
-
Disposal: Dispose of used ampoules and needles/sharps in appropriate sharps container / biomedical waste per local regulations.
Control / Regulatory Status
-
This is a prescription / physician-administered drug (not over-the-counter).
-
It may be classified as a controlled / restricted / hospital-use drug depending on national regulation.
-
Must comply with regulations for sterile parenteral products.
Quick Tips / Patient Safety Notes
-
Always have emergency reaction management ready (e.g. epinephrine, antihistamines, resuscitation equipment).
-
Infuse slowly and monitor vital signs.
-
Use diluted formulation when possible to minimize irritation or hypotension risk.
-
Document batch number, expiry, patient response.
-
Verify total iron requirement before starting so that under- or over-dosing is minimized.
-
Educate staff about signs of anaphylaxis / adverse reactions.
Your order of 100$ or more gets free standard delivery.
- Standard delivered 4-5 Business Days
- Express delivered 2-4 Business Days
Orders are processed and delivered Monday-Friday (excluding public holidays)
eMarket members enjoy free returns.
Related Products
Categories
Custom HTML Text
-
Free Delivery
From Rs 5000
-
Support 24/7
Online 24 hours
-
Free return
365 a day
- Choosing a selection results in a full page refresh.
Added to cart successfully. What's next?

Product type: 1
1 x $00.00





















 Chat with Us
Chat with Us