Your cart is currently empty.
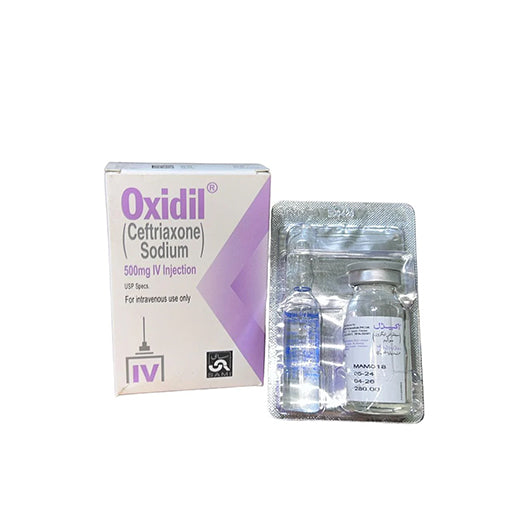
Oxidil 500 mg IV/IM Injection (Ceftriaxone) — Single Vial
: Available:
QUESTIONS & ANSWERS
Have a Question?
Be the first to ask a question about this.
Category:

Description
Oxidil injection is a sterile antibiotic preparation whose active ingredient is ceftriaxone sodium (a third-generation cephalosporin). It is supplied as powder for solution (vial) to be reconstituted for intravenous (IV) or intramuscular (IM) administration and is used to treat a wide range of bacterial infections.
Ingredients
-
Active: Ceftriaxone sodium — equivalent to 500 mg ceftriaxone per vial (after reconstitution).
-
Excipients: Typical vials contain stabilizers and are supplied with water for injection for reconstitution; check the specific manufacturer leaflet for excipient details.
Drug Class
-
Third-generation cephalosporin antibiotic (β-lactam).
Dosage Form
-
Powder for solution for injection (single-use vial). Reconstitute with the recommended diluent to prepare IV or IM dose. Available in 250 mg, 500 mg, 1 g strengths depending on supplier; this listing is for 500 mg.
Uses / Indications
Ceftriaxone (Oxidil) is indicated for treatment of infections caused by susceptible bacteria, including but not limited to:
-
Lower respiratory tract infections (pneumonia)
-
Complicated and uncomplicated urinary tract infections
-
Skin and soft tissue infections
-
Bone and joint infections (e.g., osteomyelitis, septic arthritis)
-
Intra-abdominal infections (in combination when indicated)
-
Meningitis (higher/weight-based dosing)
-
Gonorrhoea and certain sexually transmitted infections
-
Sepsis and other serious systemic infections (when pathogen is susceptible).
Dosage — Typical Regimens (Summary)
Always follow local hospital guidelines and prescriber orders. The examples below summarize commonly used adult regimens from standard references.
-
Adults (typical): 1–2 g once daily (or 1 g every 12 hours for certain severe infections). For many indications a 500 mg–1 g dose is used depending on severity and site. For the 500 mg vial, dose adjustment or multiple vials may be required to reach prescribed dose.
-
Children / neonates: Weight-based dosing — follow pediatric guidelines (e.g., up to 50–100 mg/kg/day depending on indication). Some pediatric regimens require specialized dosing for meningitis.
-
Renal impairment: Ceftriaxone is eliminated by both biliary and renal routes; dose adjustment is usually not required for renal impairment alone, but use caution and consult local guidance for severe dysfunction or combined hepatic/renal disease.
Note: Specific dose, frequency, and duration depend on infection type, severity, organism susceptibility, and patient factors. Always follow the prescriber's order and local formularies.
In Case of Overdose
-
Symptoms may include CNS disturbances, seizures (rare), and significant changes in electrolytes or renal function. Management is supportive; monitor renal function and fluid/electrolyte status. There is no specific antidote. In severe overdose, hemodialysis is not effective in removing ceftriaxone because of high plasma protein binding, but supportive and symptomatic care should be provided. Consult a poison control center or clinical toxicologist.
Missed Dose
-
Ceftriaxone is typically given as single/once-daily or scheduled infusions in a clinic/hospital. If a dose is missed, administer it as soon as possible and continue the prescribed schedule — but do not give two doses at once. Follow prescriber instructions.
How To Use / Administration Instructions
-
Reconstitution: Reconstitute powder with the diluent specified on the vial/leaflet (usually water for injection or an appropriate sterile diluent). Prepare under aseptic conditions.
-
Route: Can be administered IV slow injection or IV infusion and IM (IM route requires appropriate reconstitution and technique). Do not mix ceftriaxone in calcium-containing IV fluids (see interactions/warnings).
-
Infusion time: If given IV, follow recommended infusion times (depending on dose and local protocol). Monitor patient for allergic/hypersensitivity reactions during and after administration.
When Not to Use / Contraindications
-
Known hypersensitivity to ceftriaxone, other cephalosporins, or severe β-lactam allergy.
-
Concurrent IV administration with calcium-containing solutions in neonates (risk of precipitation / fatal reactions). Avoid co-administration of ceftriaxone and IV calcium in neonates (≤28 days).
Side Effects / Adverse Reactions
Common / less severe:
-
Injection site pain (IM), diarrhea, nausea, rash, local irritation, phlebitis (if IV).
Serious / less common:
-
Severe allergic reactions including anaphylaxis, Stevens-Johnson syndrome (rare), Clostridioides difficile–associated diarrhea, hemolytic anemia, hepatic enzyme elevations, biliary sludging (especially with high doses), and blood dyscrasias.
Important safety note: A regulatory report in Pakistan declared a batch of Oxidil substandard (February 2024) — always check batch/expiry and procure from reputable suppliers.
Precautions & Warnings
-
Administer under supervision with access to emergency treatment for hypersensitivity/anaphylaxis.
-
Use with caution in patients with history of severe penicillin allergy (cross-reactivity possible but low).
-
Monitor liver and kidney function in prolonged therapy; watch for signs of superinfection (including fungal or C. difficile).
-
In patients with biliary disease or receiving high doses, biliary sludging / gallbladder pseudolithiasis has been reported — monitor clinically if symptoms occur.
Drug Interactions
-
Calcium-containing solutions / products: Do not mix or co-administer IV calcium (e.g., Ringer’s with calcium or IV calcium gluconate/saline) with ceftriaxone in neonates due to risk of fatal pulmonary and renal precipitation; for older children and adults, avoid mixing in the same line/infusion — flush lines between administrations. Aminoglycosides: Concomitant use may increase nephrotoxicity risk — monitor renal function.
-
Oral anticoagulants: Ceftriaxone can enhance the effect of vitamin K antagonists in some patients — monitor INR.
-
Check full interaction references for other agents (hospital pharmacy/interaction databases).
Storage / Disposal
-
Storage: Store as directed on product label. Reconstituted solutions should be used according to the leaflet; many ceftriaxone powders are stable at room temperature before reconstitution — check manufacturer instructions. Store vials away from extreme temperatures and discard if vial is damaged.
-
Disposal: Dispose of unused/expired medicine and sharps per local biomedical waste regulations.
Control Drug / Prescription Status
-
Prescription only. Administered by or under the supervision of a qualified healthcare professional (hospital/clinic). Regulatory status and prescription requirements vary by country.
Quick Tips (for pharmacy/store pages)
-
Display prominently: Active ingredient: Ceftriaxone 500 mg per vial.
-
Note recommended routes: IV / IM only — not for oral use.
-
Remind buyers: Prescription required and must be administered by healthcare staff.
-
Safety callout: Do not mix with calcium IV solutions in neonates; do not use if you have severe β-lactam allergy.
Your order of 100$ or more gets free standard delivery.
- Standard delivered 4-5 Business Days
- Express delivered 2-4 Business Days
Orders are processed and delivered Monday-Friday (excluding public holidays)
eMarket members enjoy free returns.
Related Products
Categories
Custom HTML Text
-
Free Delivery
From Rs 5000
-
Support 24/7
Online 24 hours
-
Free return
365 a day
- Choosing a selection results in a full page refresh.
Added to cart successfully. What's next?

Product type: 1
1 x $00.00





















 Chat with Us
Chat with Us